Abstract
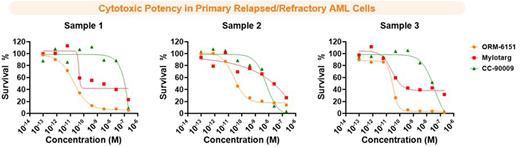
Targeted protein degradation (TPD) molecules have expanded the breadth of therapeutic options through both their catalytic mechanism of action and ability to degrade previously "undruggable" target proteins. Prior reports of small-molecule GSPT1 degraders such as CC-90009 in AML demonstrate potent anti-tumor cytotoxicity, but with a potentially narrow therapeutic index. To increase the efficacy vs. tolerability window of TPDs and improve drug delivery, we introduce TPD-Squared (TPD2TM), a dual-targeted protein degradation approach of combining the catalytic mechanism of targeted protein degradation with the precision of tumor-targeting therapeutic antibodies. We generated conjugates using a CD33-targeting antibody (OR000283) produced by engineering the FAb (H&L) sequences from gemtuzumab onto an IgG1 Fc with N297A variant to inhibit Fc-γR binding. Medicinal chemistry optimization of linker-payloads led to the identification of ORM-6151, which is composed of SMol006, a highly potent GSPT1 degrader conjugated to OR000283 via a novel β-glucoronide releasable linker. ORM-6151 treatment in CD33-expressing cell lines showed picomolar activity with 10-1000-fold greater potency compared to several GSPT1 degrader molecules including CC-90009 or Mylotarg, and had robust activity in Mylotarg-resistant lines (AML193 and Kasumi6). ORM-6151 also exhibited picomolar potency in in vitro cytotoxicity to primary relapsed/refractory AML patient blasts, with better potency than CC-90009 and Mylotarg. Moreover, ORM-6151 showed minimal cytotoxic activity to healthy hematopoietic progenitor cells, with 10-10,000 fold less toxicity than CC-90009 or Mylotarg. We evaluated ORM-6151 in several in vivo xenograft models and observed robust efficacy, following a single treatment at doses as low as 1 mg/kg. In the MV4-11 xenograft model, treatment with ORM-6151 demonstrated superior activity than CC-90009. The tumor growth inhibition correlated with the degree and duration of GSPT1 depletion and changes in expression of previously described integrated stress response biomarker genes. In summary, ORM-6151 is a promising, potential therapy for AML and currently in preclinical development as a first-in-class targeted protein degrader therapy with CD33-targeted delivery.
Disclosures
Palacino:Orum Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Lee:Orum Therapeutics: Ended employment in the past 24 months. Jeong:Orum Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Kim:Orum Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Song:Orum Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Permpoon:Orum Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Wong:Orum Therapeutics: Ended employment in the past 24 months. Bai:Orum Therapeutics: Ended employment in the past 24 months. Fishkin:Orum Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Takrouri:Orum Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Yu:Orum Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Yi:Orum Therapeutics: Ended employment in the past 24 months. Skaletskaya:Orum Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Chang:Orum Therapeutics: Ended employment in the past 24 months. Kim:Orum Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Kim:Orum Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Choi:Orum Therapeutics: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Park:Orum Therapeutics: Current Employment, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal